The latest investigations by the Research Centre for Fire Protection Technology at the Karlsruhe Institute of Technology show that: If hydrogen is released from a hydrogen-powered vehicle in an underground car park, it quickly dissipates from the structure under favourable structural conditions. Positive pressure ventilation can hardly accelerate this process in the special situation investigated. However, it would probably not pose a hazard under these conditions, as positive pressure ventilation does not flood previously uncritical areas with ignitable hydrogen-air mixtures.
Conduct forced ventilation in case of hydrogen release without a fire?
Many fire services are currently working on the further development of standard operational procedures for incidents involving hydrogen-powered vehicles, which we have reported on continuously in our magazine. The question arose as to whether tactical ventilation makes sense when hydrogen is released in an underground car park without a fire or can even lead to an increase in the risk of explosion.
Summary of the specialist presentation from the Fire Chief Forum 2024
The Research Centre for Fire Protection Technology at the Karlsruhe Institute of Technology is investigating this issue as part of the fire protection research of the German federal states (D) on behalf of the Committee for Fire Service Affairs, Civil Protection and Civil Defence. Dietmar Schelb presented the initial results of his flow simulations with the CFD programme Ansys CFX (CFD stands for Computational Fluid Dynamics) at the International Fire Academy's 2024 Fire Chief Forum. Together with Dietmar Schelb, we have prepared the following summary.
Analysed scenario with structurally favourable conditions
For the CFD simulation, it was assumed that 6 kg of hydrogen discharges diffusely from a pressurised vehicle tank within 60 s, that the ceiling of the underground car park is flat and has no beams and that the structure has an entrance and exit on the opposite side where the hydrogen-air mixture can flow unhindered into the open air.
Rapid dissipation of the hydrogen-air mixture, even without forced ventilation
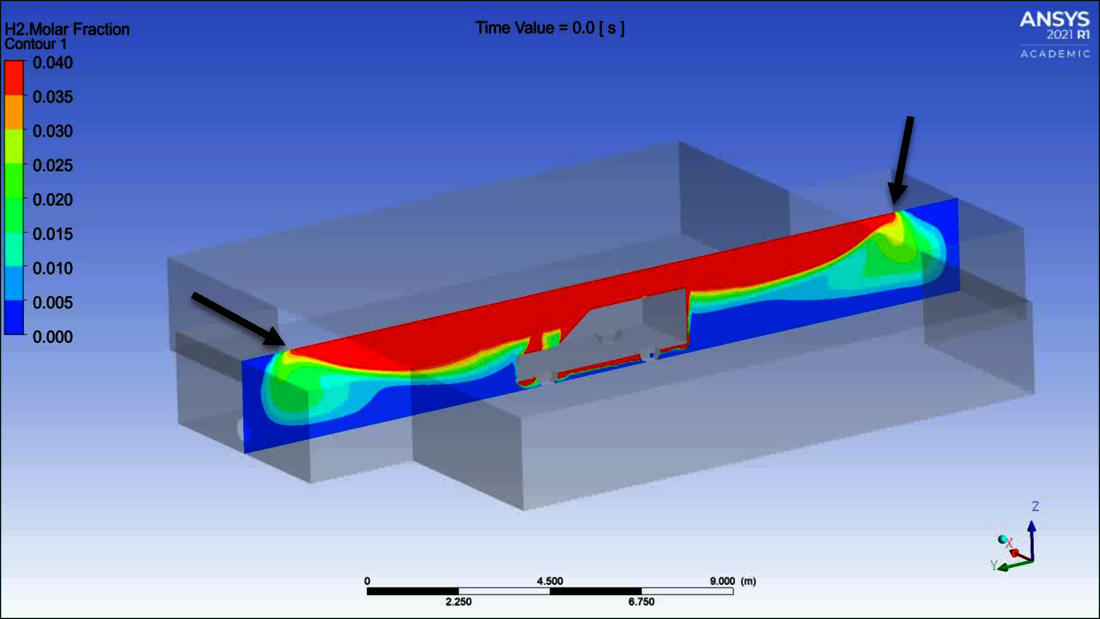
Diagram 1 shows a longitudinal cross-section with the concentration of hydrogen in the air at the time immediately after the end of the discharge from the pressurised container. Hydrogen rises out from under the vehicle, and the light hydrogen-air mixture flows to the right and left along the ceiling through the entrance and exit to the outside (black arrows). A «molar fraction» value of 0.04 means 4 % hydrogen in the air. It is the lower explosion limit. The scale is designed so that mixtures from just below the lower explosion limit to above the upper explosion limit are shown in red.
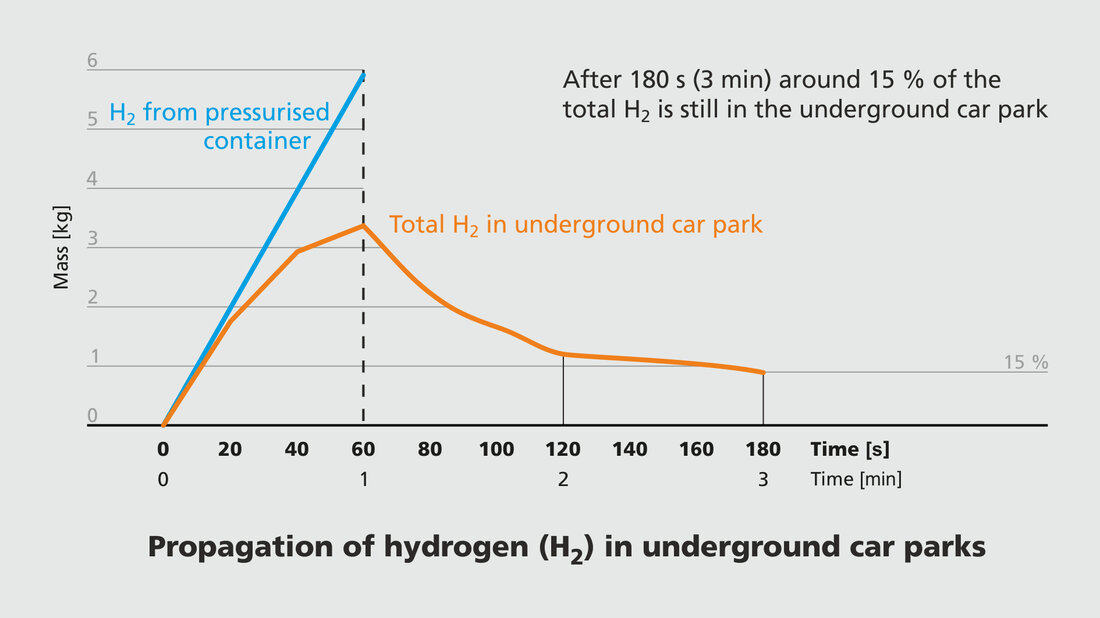
In diagram 2, the blue straight line shows that the 6 kg of hydrogen is released after 60 s. The orange curve shows that after these 60 s, almost half of the hydrogen released from the pressurised container dissipated into the open. After 60 seconds, 20 % of the released hydrogen (less than one kilogramme) is still distributed in the underground car park in a low mixing ratio (below the lower explosion limit). And after a total of 180 seconds, just 15 % remains.
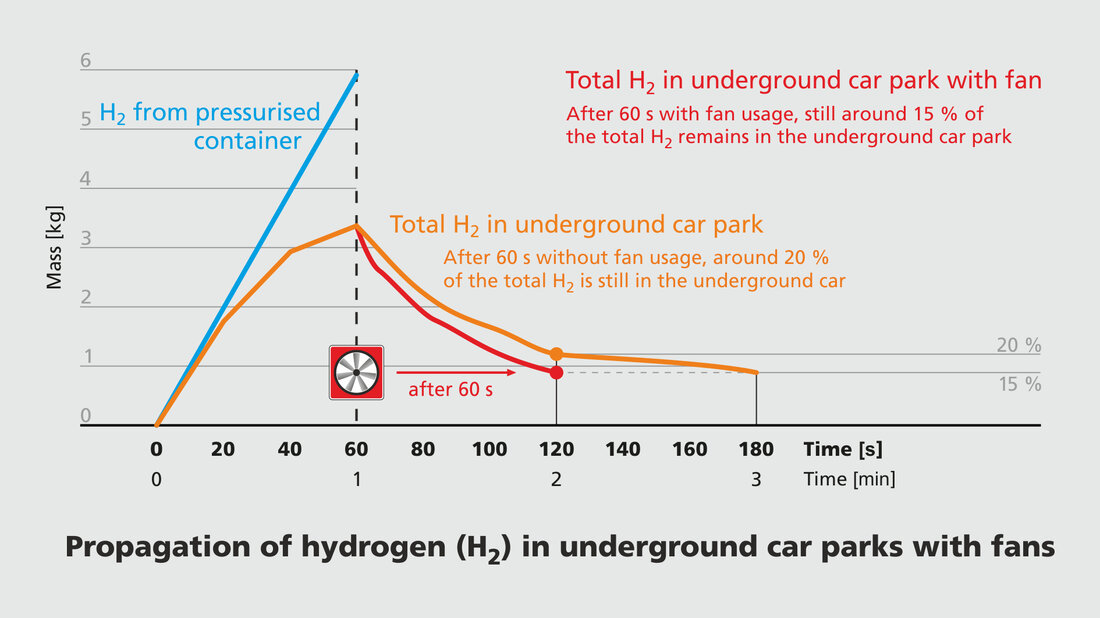
Fan only has a minor effect
In a second CFD simulation, the effect of a fan blowing air at an angle of 20 degrees to the ceiling for 60 s, starting immediately after the discharge from the pressure vessel, was investigated. However, this only slightly reduces the amount of hydrogen present in the underground car park.
Early use of fans rather unrealistic
Initially, the simulation was designed so that the positive pressure ventilation would only start after a realistic arrival and set-up time of the fire service of 10 to 15 minutes after the hydrogen had escaped from the pressurised container. However, the simulation showed that after 10 minutes, practically all of the hydrogen had already dissipated into the open air, even without a fan. To investigate the effect of positive pressure ventilation, positive pressure ventilation had to be started at an unrealistically early point in terms of operational tactics.
Still many unanswered questions
Of course, the CFD simulations carried out do not reflect real operating situations. The aim was to recognise fundamental correlations. From the fire services' point of view, this could lead to the conclusion that forced ventilation is of little use, as the flammable hydrogen-air mixture dissipates very quickly anyway. However, this is only the case if there is an exhaust air opening to the outside at the highest point of the underground car park. According to Dietmar Schelb, the next step will be to investigate how pressurised ventilation affects hydrogen-air mixtures in ceilings with obstructing beams, as light gas mixtures can accumulate there.
Fan useage probably does not increase the risk of explosion
Given the current state of knowledge, Dietmar Schelb considers it unlikely that the fire service could increase the risk of an explosion using positive pressure ventilation under these specific structural conditions, where the hydrogen can quickly escape into the open. It is an entirely different question whether fire services can arrive at the scene and ventilate before the hydrogen has escaped from the building.
No consequences for tactical ventilation in the event of a fire
To avoid any misunderstandings, the CFD simulations reported here relate solely to the release of hydrogen without fire. In the event of a fire, any hydrogen present would burn and the usual tactical ventilation can be used to dissipate heat and smoke.
Update will follow
We will continue to report on operationally relevant findings from current hydrogen safety research. Dietmar Schelb will be happy to provide detailed information on the latest research results, especially for colleagues working in preventive fire protection.





